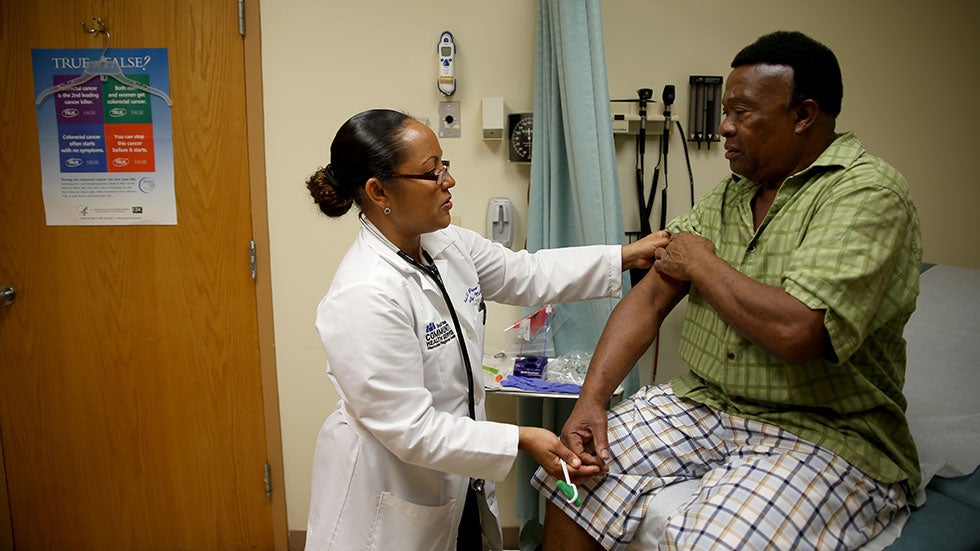Bringing a new drug, therapy, or medical device to market is a time-consuming and cost-intensive process. In fact, the average clinical trial takes six or seven years to complete with a typical price tag of $2.6 billion.
Introducing the Reinvented FSP: Functional, Flexible, and Fractional Service Provider
Topics: clinical trial, clinical trial strategic planning, clinical trial consulting, clinical trial remote services, data mangement, Clinical Research, Biotech, Pharmaceuticals, biotechnology, pharma, qualityassurance, biostatistics, biometrics, FSP, Functional Service Provider, clinical trials, project management, Good Clinical Practice, patient enrollment, FDA inspections
3 Common Clinical Trial Models and Tips for Choosing the Right One
The last year and a half have been a whirlwind, particularly for clinical researchers. While the trial landscape is ever-evolving, efforts to “slow the spread” of COVID-19 have accelerated the process.
Topics: clinical trial, clinical trial strategic planning, clinical trial consulting, clinical trial remote services, Clinical Research, Biotech, Pharmaceuticals, biotechnology, biostatistics, biometrics, FSP, clinical trials, online meetings, project management, Good Clinical Practice, patient enrollment, FDA inspections
4 cost-effective strategies for recruiting clinical trial participants
Designing and implementing a clinical trial is a time-consuming and costly process. In fact, a 2014 report by Aylin Sertkaya et al. submitted to the U.S. The Department of Health and Human Services found that on average:
Topics: clinical trial strategic planning, clinical trial consulting, clinical trial remote services, Clinical Research, Pharmaceuticals, clinical trials, patient enrollment, patient recruitment
The expanding Coronavirus (COVID-19) global crisis has governments, companies and people implementing plans to respond, meanwhile, governments and businesses still must run, and people still need to lead their lives. At Harbor Clinical, we’re prepared for events like this by having effective plans to work remotely in place.
Topics: clinical trial strategic planning, clinical trial consulting, clinical trial remote services