As sponsors continue to restart paused studies, there will be a flurry of medical writing activities taking place to support IND submissions, investigator brochures, protocols, and other documents. Given that, below are tips to help improve the quality and efficiency in creating these documents.
Recent Posts
7 Tips for Enhancing Medical Writing Quality and Efficiency
Topics: clinical trial, fda, Clinical Research, Biotech, Pharmaceuticals, biotechnology, medical writing
by Larry Claflin Jr.
Planning a vacation in the middle of a global pandemic with limited travel options?
Topics: Clinical Research, Pharmaceuticals, biotechnology, vacations
Edit check programming and testing is time consuming and labor intensive. As such, it is efficient to minimize the time spent on programming by getting it right the first time. Historically, data management have measured edit check success as the percentage of edit checks that pass User Acceptance Testing (UAT). However, that may not be the best measure. What if an alternative metric leveraging Lean Six Sigma Methodology was used? The Rolled Throughput Yield (RTY) is a metric often employed in manufacturing operations to detect the probability that a process with more than one step will produce a defect free unit. If we track this metric for the edit check process, we can better gauge the probability of success of the edit check program.
Topics: clinical trial consulting, Data Management, Database Development, Clinical Research, Biotech
Vendor Oversight: Not a New Normal, Just an Interim Normal
Given our recently-modified approach to executing clinical trials due to the COVID-19 pandemic, it is critical that we update our vendor oversight approach, ask key questions of our vendors, and modify our strategy based on the answers to these questions. Harbor Clinical has the experience, expertise, and plan to assist you in adapting your vendor oversight quickly and effectively.
Topics: clinical trial strategic planning, clinical trial consulting, fda, inspection readiness, clinical trial inspection
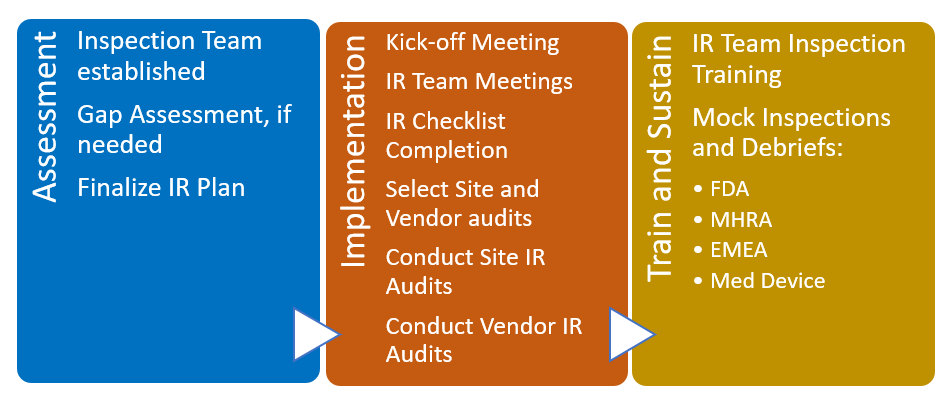
Inspection Readiness - A Critical Success Factor in Clinical Trials.
A consistent challenge in conducting clinical trials of new pharmaceutical products is staying in compliance with governmental requirements. Governmental organizations such as the FDA in the United States conduct inspections with the purpose of verifying that the clinical trial is adhering to the requirements and standards in force. The FDA, for example, will conduct inspections for the one or more of the following reasons:
Topics: clinical trial strategic planning, clinical trial consulting, fda, inspection readiness, clinical trial inspection
The expanding Coronavirus (COVID-19) global crisis has governments, companies and people implementing plans to respond, meanwhile, governments and businesses still must run, and people still need to lead their lives. At Harbor Clinical, we’re prepared for events like this by having effective plans to work remotely in place.
Topics: clinical trial strategic planning, clinical trial consulting, clinical trial remote services
At Harbor Clinical, we provide expertise to small/mid-sized biotech/pharma companies to ensure the best possible outcomes of the clinical trials that are crucial to their company’s success. We provide subject matter experts across functional areas including quality assurance, vendor oversight, clinical operations, medical writing, regulatory, biometrics and strategic development.
Topics: clinical trial, clinical trial strategic planning, strategic planning, clinical trial consulting