A consistent challenge in conducting clinical trials of new pharmaceutical products is staying in compliance with governmental requirements. Governmental organizations such as the FDA in the United States conduct inspections with the purpose of verifying that the clinical trial is adhering to the requirements and standards in force. The FDA, for example, will conduct inspections for the one or more of the following reasons:
- to verify the accuracy and reliability of data that has been submitted to the agency
- as a result of a complaint to the agency about the conduct of the study at an investigational site
- in response to sponsor concerns
- upon termination of the clinical site;
- during ongoing clinical trials to provide real-time assessment of the investigator’s conduct of the trial and protection of human subjects at the request of an FDA review division
- related to certain classes of investigational products that FDA has identified as products of special interest in its current work plan (i.e., targeted inspections based on current public health concerns).
As with any activity, preparation is always the key to success, and the same is true with a clinical trial inspection. Harbor Clinical has found that one of the best methods of preparation is to conduct a thorough readiness review and a mock inspection of a client’s clinical trial. Conducting the readiness review and mock inspection with the same rigor as the inspecting agency will ensure that the client is ready when the real inspection occurs.
Harbor Clinical’s Inspection Readiness practice achieves the following objectives:
- Assemble a client Inspection Readiness (IR) Team
- Prepare the client IR team for inspections by the FDA, EMA and MHRA
- Ensure key documents and records are easily accessible and compliant
- Identify any gaps in compliance and provide a remedial solution
- Select a risk-based sample of the client’s vendors and sites to:
- Evaluate Inspection Readiness
- Ensure compliance with selected protocol(s)
- Identify any gaps in compliance with GxP and regulatory requirements
- Lead efforts to develop and execute a corrective and preventive action (CAPA) plan
Our plan for accomplishing the objectives follows three phases:
- Assessment
- Implementation
- Train and Sustain
The graphic below explains how the program works:
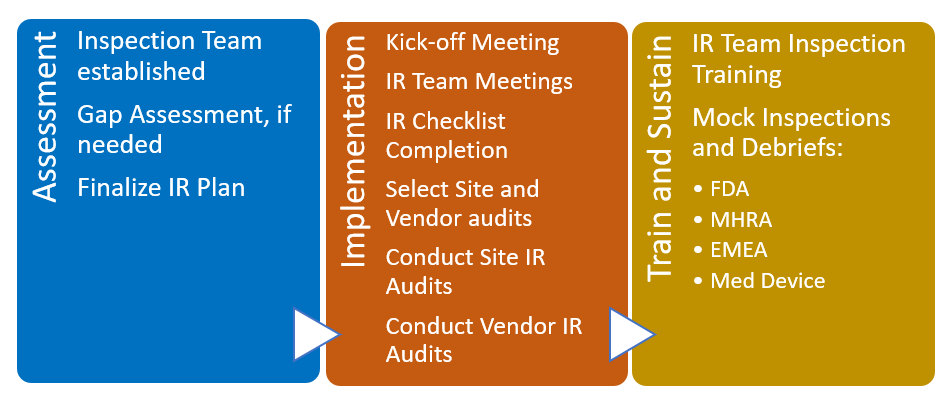
Assessment Phase: In the assessment phase, we partner with the client in identifying the inspection readiness team from their employees teamed with a Harbor Clinical Auditor(s). Using a train-the-trainer model, this team is involved throughout the inspection readiness process. Harbor Clinical, with the client will develop, review and finalize the Inspection Readiness Plan. Additionally, and if needed, this phase also includes an assessment of the Quality Management System to spot gaps in compliance or procedures. Any identified deficiencies will be addressed prior to the next phase, Implementation.
Implementation Phase: In this phase we ensure the team is prepared for the mock inspections and an actual inspection. We complete a risk assessment to determine which sites and vendors should be audited and to what extent. The Implementation and Train and Sustain Phases overlap somewhat – the training is ongoing and scheduled when convenient to the team. Likewise, site and vendor audits are scheduled based on availability and risk assessment versus any specific sequence.
Train and Sustain Phase:
In this phase, Harbor Clinical conducts the actual mock inspections to prepare the client team for the actual inspections from regulatory authorities. The training programs are thorough and well documented therefore the best preparation is a comprehensive “dress rehearsal” by conducting the mock inspection. We seek to conduct our mock inspections with the highest fervor to replicate the real inspections, giving the team adequate preparation time prior to each mock inspection, training and compliance assessment during the mock inspection itself, and abundant post-inspection follow up to the client is optimally ready for their inspections.
Learn More!
Harbor Clinical’s Inspection Readiness practice is the best way to ensure your teams are prepared for regulatory organization inspections. Click this big blue button to let our team know you'd like to talk about this key issue.




