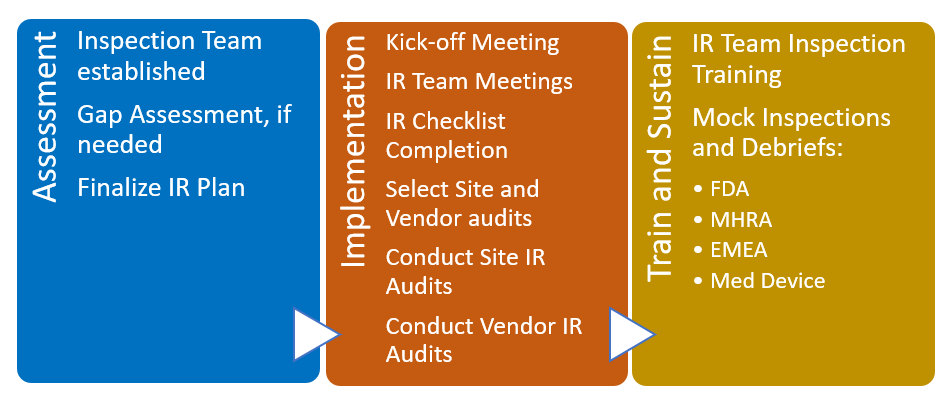
A consistent challenge in conducting clinical trials of new pharmaceutical products is staying in compliance with governmental requirements. Governmental organizations such as the FDA in the United States conduct inspections with the purpose of verifying that the clinical trial is adhering to the requirements and standards in force. The FDA, for example, will conduct inspections for the one or more of the following reasons:
Inspection Readiness - A Critical Success Factor in Clinical Trials.
Posted by
Ann Conner on May 14, 2020 6:45:30 PM
0 Comments Click here to read/write comments
Topics: clinical trial strategic planning, clinical trial consulting, fda, inspection readiness, clinical trial inspection