An earlier blog had identified the benefits of using Earned Value Management (EVM) techniques in Clinical Studies. This synopsis provides a practical example of how to implement those techniques and making useful decisions from the available data. To support this example, a mock study with the following scope of work, budget and timeline requirements has been established:
The study budget is separately categorized for Data Management activities and Clinical Trial Monitoring activities. The baseline start and finish dates define the project requirements to be deemed as “On Track” for schedule and cost. If the schedule of tasks and activities listed in the table above start and finish within those baseline dates, the budgeted work is “earned”. The definitions of EV Measurement Technique used for assessing performance are as follows:
Level of Effort (LOE): this technique is applied when an activity spans across a large time duration and in general does not have discrete deliverables. This technique provides an equal schedule value earned for every dollar expended in a particular reporting period. Typically use of LOE as measurement techniques should
represent no more than 20% of the total project budget. If the total LOE tasks on a project were say 80%, the true indicator of a schedule or cost erosion would be masked. In each period schedule performance earned would equal actual cost expended, resulting in the Schedule Performance Index (SPI) or Cost Performance Index (CPI) to be 1.0. This would not be a very informative assessment of how a project is executing.
50/50: this technique is applied when an activity spans 2 months in duration. 50% of the schedule earned occurs when the activity starts and 50% is earned when a project completes.
0/100: this technique is applied when an activity spans less than 1 month in duration. If an activity is started in one month, but is not finished until the second month, the metrics are unfavorably affected. CPI will be unfavorable as no work was completed though costs were incurred. Since no work was performed SPI will be 0.0 in that month.
% Complete: this technique is applied when a long term activity, longer than 2 months exists and has multiple intermediate discrete tasks which can be an indicator of project performance. There is nothing that prevents the Project Manager to plan each of these discrete tasks into shorter durations whereby eliminating the need for using this technique. Sometimes there can be project uncertainties which cannot be planned out in advance and as such use of a % complete worksheet allows for some built in flexibility. This metric requires creating a standalone worksheet which defines discrete tasks for which budget is allocated as well as start and finish projected dates.
With the project details defined and the measurement techniques for tasks established, the project has commenced. An assessment of the project for month end July-2020 is now being assembled for an upcoming presentation to leadership. The project manager has updated the project status for the respective tasks and has quantified the metrics that will be reported: CPI and SPI. This requires clarification of some terms:
- Budgeted Cost of Work Scheduled (BCWS) – this is the work baselined to complete as of the reporting period
- Budgeted Cost of Work Performed (BCWP) - this is the work performed as of the reporting period
- Actual Cost of Work Performed (ACWP) – this is the cost expended to complete the work (BCWP) as of the reporting period
CPI = BCWP/ACWP
SPI = BCWP/BCWS
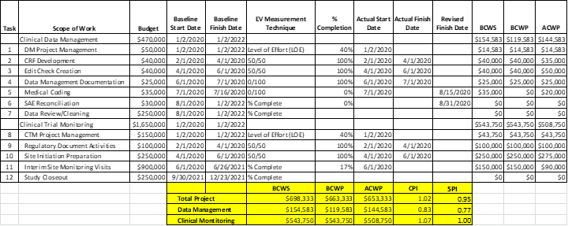
From the table above, the project CPI is favorable (CPI of 1.02- below budget), but is behind schedule with an SPI of 0.95 (1.00 would be deemed on schedule). When looking at the contributors to the performance, Data Management CPI and SPI are unfavorable as of this reporting period. An assessment of this performance indicates that Medical Coding (Task 5) did not complete in July resulting in an unfavorable schedule variance of $35,000 (from a BCWP to BCWS comparison). Cost was expended in the period, but the activity did not complete and as such no schedule progress was earned. On the Clinical Monitoring side, cost performance is favorable (under budget), with a CPI of 1.07, and is on schedule with an SPI of 1.00. An assessment of this pattern indicates that Site Initiation preparation (Task 10) was over its cost budget, however that was easily recovered as progress for Interim Site Monitoring Visits (Task 11) was completed at a very favorable cost in the reporting period (cost variance of $60,000 from a ACWP to BCWP comparison). While this evaluation and assessment provide a good synopsis of what happened, the Project Manager’s challenge is to determine recovery options from these setbacks, if possible. Can the unfavorable SPI be recovered in the subsequent reporting periods? Will data management activities experience continued cost growth? Will the favorable cost performance trend for Clinical Monitoring continue to compensate for the Data Management overrun?
Performing regular project status updates, as identified in this example, on a monthly basis provides a usable historical data set which offers the following benefits:
- Financial Accruals - actual study costs can be tracked on a monthly basis providing insightful data to the finance organization
- Scope Management – Tracking deliverables against established milestones to proactively monitor any deviations which would result in costly Change Orders that could be received unfavorably
- Cost V. Budget Tracking – In a Functional Source Provider (FSP) model, this data allows for tracking FSP spend against the budgeted spend to compare the initial resource forecast to actual for resource balancing purposes
Implementing EVM techniques provides more insight to assess challenges experienced during the project and better prepares the organization to manage financial outcomes of the clinical study.




